Project Introduction
How can I stand on the ground every day and not feel its power? How can I live my life stepping on this stuff and not wonder at it?
B. Logan (1)
The problem of industrial agriculture
How will we grow food for an expanding global population while supporting the resilience and integrity of the biosphere? This is a defining question of the Anthropocene.
The experiment of agriculture began approximately ten thousand years ago when humans started to domesticate wild annual plants. The selection of annuals constrained agricultural innovation and necessitated the development of novel and radically simplified food production systems characterized by frequent anthropogenic disturbance (2). When we flash forward to the present, we see that this pattern of ecological simplification and frequent disturbance has reached a zenith. Since the advent of the fossil fuel economy and the Green Revolution in the mid-20th century, intensive industrial systems of food production have emerged as the dominant agricultural paradigm. Industrial agriculture is characterized by practices such as annual monocropping of cash crops, frequent tillage, fallow winter fields, and heavy reliance on fossil fuel-generated pesticide and fertilizer inputs (3).

Image 1. Intensive Industrial Agriculture
Intensive industrial agriculture seeks maximal control over food production, endeavoring to transform a process that is foundationally biological into a highly regulated industrial input and output factory. Industrial agriculture is made of a matrix of relationships of domination, coined by scholar Timothy Morton as agrilogistics (4). Technical, mechanized, and methodical, agrilogistics separates humans from more than human worlds. A top-down movement, the industrial agriculture behemoths generated a ‘one size fits all’ method of food cultivation, a program that spread across (colonized) the globe.
At first, adoption of industrial agriculture boosted productivity as measured by yields—global crop yields increased by 56 percent between 1965 and 1985 and then by 20 percent between 1985 and 2005 (5). However, in the past decade, crop yields have plateaued or declined (6) signaling that agrilogistics is faltering—we are at or just past the peak (peak fossil fuel, peak water, peak grain production, peak phosphorous, etc.) and now find ourselves in a century of decline and ecological devastation (7).
This faltering precipitated from agrilogistics’ bulldozing over the nuances of local ecologies. By ignoring ecological reality, industrial farming has inflicted widespread damage. Whereas natural ecosystems support life through ecosystem functions and services, industrial agriculture degrades biodiversity and generates a suite of ecosystem and societal disservices (8). Prominent disservices generated by industrial agriculture include biodiversity decline (9), soil erosion (10–12), greenhouse gas emissions (13), nutrient contamination (14, 15), antibiotic resistance (16), corporate consolidation (17), pesticide treadmills (18), human health inequities (19), and the emergence of epidemics and pandemics (20).
Industrial agriculture and the unseen majority
“The microscopic majority can no longer be the unseen elephant in the room.”
Cavicchioli et al.(21)
One often overlooked harm of industrial agriculture is the disruption of soil microbial life. Increasingly intensive land use threatens soil biodiversity globally (23). Dark, opaque, and inherently complex, biological knowledge characterizing the “black box of soil” (24) is exceptionally difficult to generate. But the more we learn about soil microbes, the more we recognize their centrality to agriculture (25), human health (26), and all life on earth (27).
The hidden world of soil microbes is almost unfathomably diverse and strange. Soils are a complex and dynamic reservoir of microbial biodiversity. A single gram of productive soil contains between 100 million to one billion organisms and thousands of individual taxa spanning all three domains of life: Archaea, Bacteria, and Eukarya (27). The living soil food web includes bacteria, fungi, archaea, protists, algae, nematodes, and other microbial eukaryotes (28).

Image 2. A Handful of Healthy Soil
This invisible and astounding microbial diversity constitutes the foundational life support of the Earth. Microbes date back 3.8 billion years to the origin of life on our planet (29). Positioned near the base of the food web, they provide essential support for all higher trophic levels. Microbial activity is critical for maintaining global biogeochemical cycles and the integrity of terrestrial and marine ecosystems (29). Microbes are ubiquitous—all macrorganisms are covered by a surface of microbes, an invisible second skin. The microbiome is a core component of an organism’s ecophysiology and identity (30). It is hard to overstate the centrality of microbes to supporting life in the biosphere as we know it.
Although all macroscopic organisms live in intimate and dynamic relationship to microbes, until recently, they have been all but ignored. We have overlooked microbes in large part due to the basic reality that they are not observable to the naked eye—they are functionally invisible without the assistance of technology.
As a discipline, microbial biology is a young and dynamic field with more questions than answers (31). Scientists are still debating the definitions of fundamental microbiological terms such as microbiota and microbiome (32, 33). The term microbiome was only first coined by Whipps et al. in 1988 and defined as “a characteristic microbial community occupying a reasonably well-defined habitat which has distinct physio-chemical properties. The term thus not only refers to the microorganisms involved but also encompasses their theatre of activity (34).”
While there are substantial data gaps in microbial knowledge, there have been massive strides in our understanding since the advent of next-generation sequencing technologies, such as metabarcoding and shotgun sequencing (28). These molecular technologies have allowed scientists to characterize soil microbiomes at new depths, deciphering complex processes that govern the assembly and functioning of microbiomes (35).
There is urgent need for concerted soil biodiversity conservation efforts
“We know more about the movement of celestial bodies than about the soil underfoot.”
Leonardo Da Vinci, circa 1500s
Soil, the habitat for soil microbiota, is rapidly eroding due to agricultural intensification (10). Soils are the principal reservoir for biodiversity in terrestrial ecosystems. They harbor approximately one quarter of all biodiversity on Earth (36). Soil microbes are canaries in the coal mine—when microbial populations begin to disappear, ecosystem multifunctionality is soon to follow (36). As soil biodiversity changes in response to land use, there are far reaching and unpredictable results (37). The erosion of soil and the extinction of complex belowground microbial diversity impairs the functionality of earth systems (29). The living soil regulates many critical aboveground processes (36). Living soil provides humanity with 98.8 percent of its food (10). Soil security is a necessary prerequisite for sustained food security.
Despite these high stakes, it is easy to overlook belowground extinction events. Recent calls to expand environmental protection overlook soil microorganisms (38). There is low motivation for microbial conservation, especially when compared to plants and animals, particularly charismatic macrofauna (39). When soils are considered by conservationists or environmentalists, there is emphasis on the chemical and physical properties, while the soil biology is most often overlooked (40).
Since the baseline biodiversity of the “black box of soil” (24) is poorly characterized, it is challenging to quantify biodiversity losses or measure conservation success (52). We are tasked with the challenge of conserving an enormous reservoir of biodiversity that is largely unseen and largely unknown. For instance, fungi outnumber plants by at least six to one (53). Estimates of fungal diversity range broadly from 1.5 to 10 million species (54). Today, the most commonly accepted range is between 2.2 to 3.8 million fungal species (54). Since 120,000 fungal species are currently classified, we have only named at best eight percent and at worst three percent of fungal species (54). These gaps in our knowledge of fungal diversity and microbial diversity limits our awareness of belowground extinctions and extinction risk (55). We are rapidly losing microbial biodiversity that is neither known nor quantified.
Gopal and Gupta 2019 submit that there is urgent need to establish a plant microbiome vault. Similar in concept to the Svalbard Global Seed Vault, this plant microbiome vault would assist research, help to ensure long term food security, and improve intergenerational equity by preserving plant microbiomes in perpetuity (56). Additionally, a group of international microbiologists recently established the Soil Biodiversity Observation Network (SoilBON) so as to more systematically sample and monitor changes in soil biodiversity worldwide. This monitoring effort aims to impact policy by empirically demonstrating the dire need for microbially protective interventions. Other international scientific endeavors, such as the Global Soil Biodiversity Initiative and the Food and Agriculture Organization’s Global Soil Partnership, are tirelessly working to build capacity and raise awareness about the importance of soil biodiversity.
One illustrative example of how large data gaps in microbiological knowledge impact policy creation is the European Union’s proposed 2006 Soil Framework Directive. The directive explicitly acknowledged that loss of soil biodiversity is a primary factor degrading European soil fertility. Despite this, the directive concluded that authoritative scientific knowledge on the topic of soil biodiversity was “too limited to allow for specific provisions … aiming at its protection” and the directive was subsequently withdrawn (51). If scientists are still discovering and debating questions of microbial biodiversity, it is no surprise that policy makers and the general public are undereducated and unmotivated to conserve microbial life.
Microbes confer many benefits to agroecosystems & farmers
“Essentially, all life depends upon the soil … There can be no life without soil and no soil without life; they have evolved together.”
Charles E. Kellogg, USDA Yearbook of Agriculture, 1938
A foundationally biological endeavor, agriculture exists in intimate relationship with microbes. Industrial agriculture has largely ignored the beneficial contributions of microbes to its own detriment, instead shortsightedly focusing on eradicating pathogenic microbes. To facilitate transition from agrilogistics to an ecologically literate agriculture, we must first uncover and then digest the centrality of the soil microbiome in agriculture (36).
Plants and their microbiomes co-evolved (37). It is now thought that mitochondria and plastids (intracellular plant organelles) evolved from endosymbiotic bacteria (38). And it was arbuscular mycorrhizal fungi and host plant mutualism that facilitated plant terrestrialization 460 to 480 million years ago (39, 40). Plants selectively assemble their root microbiomes from the surrounding soil microbiome (41). Using structural modifications and chemical signaling, plants modulate the microbes found in the rhizosphere, the narrow zone of soil surrounding the root (41). Logically, the composition of the soil microbiome constrains the design of the plant root microbiome (36).
Plant roots deposit between 10 to 44 percent of their photosynthetically fixed carbon into the rhizosphere (42). In return for photosynthetic carbon input, the plant microbiome offers myriad benefits to their host including acquiring essential plant nutrients and water, fixing atmospheric nitrogen, promoting plant growth, protection against infection, and defense against predators (30). Additionally, through decay of soil organic matter, the soil microbiome improves soil fertility and soil structure, detoxifies heavy metal contamination, and enhances carbon storage capacity, indirectly bolstering plant fitness (25, 43). While dominant agricultural practices today overlook the importance of microbial stewardship, current research unambigously demonstrates that microbes offer many fitness advantages to plants in agroecosystems.
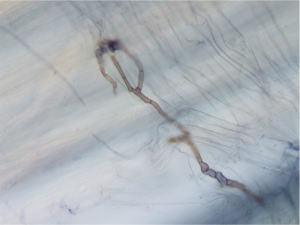
Image 3. Mycorrhizal Fungi in a Plant Root
In recognition of the importance of soil microbes to plant development, growth, and fitness, plant microbial genomes are now referred to as the “second genome” of plants (44). Microbiology research has brought on a major shift in our concept of what a plant is. We now classify plants as holobionts, an assemblage of plant and microbes forming a discrete and interdependent ecological unit (45). The holobiont concept is a new vision that challenges biologists to upgrade fundamental theories. Positioning microbes as centrally important for understanding plant biology, under the holobiont theory “plants are no longer heralded as autonomous entities, but rather as biomolecular networks composed of the host plus its associated microbes” (46).
Industrial agriculture disrupts microbial life
Although we do not know the entire scope of how industrial agriculture has impacted and is impacting soil biodiversity globally, we do know that various agricultural practices differentially impact microbiomes. Plant microbiomes are sensitive to physical, chemical, and biologicals stressors. Globally, agriculture is a principal source of abiotic and biotic stress on microbial communities. Agriculturally intensive practices reduce overall biodiversity (57, 58) and shift the balance of soil microbial populations and trophic groups (52). Tillage, nutrient addition, agrochemical inputs, and other agricultural disturbance create biotic and abiotic filters on microbial communities, establishing strong selective pressures where some microbes thrive and other diminish (59–61). For instance, low input (extensive) management of agricultural land results in fungal-based soil food webs while intensively managed land has bacterial-based food webs (62). Soils with higher disease suppressiveness rates are associated with higher fungal diversity (28).


Image 4. Industrial Agriculture Simplifies Soil Microbiomes
Changes in the balance of microbial populations support disease emergence: “The main reason for the high incidence of soil-borne diseases in croplands is the deterioration of the micro-ecological environment that can destroy or alter the balance of the soil microbial communities” (28). Intensive management combined with short crop rotations encourages accumulation of pathogenic microbes which substantially reduce yield (36). Farmers combat soil borne pathogens by applying health hazardous pesticides such as methyl bromide, chloropicrin, and 1,3-dichoropropene (18). Pesticide is a general term for any chemical used to kill a ‘pest.’ They are many classifications of soil targeting pesticides: fungicides (kill fungi), bactericides (kill bacteria), insecticides (kill insects), algaecides (kill algae), virucides (kill viruses), nematicides (kill nematodes), acaricides (kill mites), etc. (63).

Image 5. Fumigants in Use
Agricultural reliance on pesticides to kill microbes is an extension of the industrial pattern of simplification born out of ignorance of ecology and a desire for complete control over biological development. As part of the project of simplification, human-engineered industrial food systems create conditions that divorce plants from their microbial partners and erase the centrality of microbes to plant development. For instance, when a plant is regularly showered with NPK (nitrogen, phosphorus, potassium) fertilizer, it has less incentive to associate with mycorrhizal fungi—why would a plant siphon off carbon to nutrient scavenging fungi when these essential nutrients are readily available in the soil profile? These hyper simplified systems displace microbial relationships that sustain plant communities with nonrenewable fossil fuels. Continuing the above example, production of NPK fertilizer is fossil fuel intensive: “Of the energy required to ‘farm’ a typical acre of maize in the USA today, 99.95 percent of the calories used originate from fossil fuels” (2). Ultimately, this reliance on fossil fuel generated agrochemicals to control pathogens creates soil dysbiosis—it shifts soils from disease suppressive to disease conducive (28).
Agrilogistics’ erasure of the centrality of microbes to sustaining agroecosystems is dangerous and ultimately impossible to maintain. Downstream, these simplified systems backfire when pathogens emerge and the plants are vulnerable and functionally naked, without protection from microbial partners. Ironically, by trying to reduce stress and system complexity for their plants of interest (crops), industrial agriculture creates an ecologically brittle system primed for the emergence of novel soil borne pathogens. As the development of microbial resistance demonstrates, there is a natural expiration date to the success of pathogen management through chemical warfare (16). Ultimately, by eradicating non-target microbes (not to mention insects (64), birds (65, 66), stream invertebrates (67), amphibians (68), and mammals (69), including humans (70–74)) and disrupting the balance of soil microbiomes, pesticide application creates more problems than it solves (75). A major project of agriculture in the 21st century is to integrate the holobiont concept into agricultural crop management, creating contexts where plants and their microbiomes are in dynamic and continual interchange.
Farmer deskilling and ecological illiteracy
“[Farmers] know that legumes are nitrogen fixing plants. But what is not always know is that it is really the microbes associated with the legumes that are doing the fixation.”
Kristin Veum, PhD, USDA-ARS Soil Microbiologist (76)
In 1904, Lorentz Hiltner, a prominent German microbiologist who coined the term rhizosphere, asserted, “I am convinced that soil bacteriology will finally provide results that are not only of explanatory nature but will also directly affect and determine agricultural practice” (77). We have not yet reached Hiltner’s projected future. Although our understanding of soil microbiology has greatly expanded over the past century, microbiological and ecological concepts are not taught to farmers or integrated into agriculture as it is widely practiced today. While most farmers know that legumes are nitrogen fixing, many farmers do not understand that it is microbes, not the plant, that are introducing nitrogen into the system. It is well past time to close this knowledge gap.
There is a pervasive societal microbiophobia (germaphobia) that perceive microbes as bad actors (a fear enforced by the COVID-19 pandemic) (30). Advertising campaigns depict microbes as dangerous and hostile—they must be entirely “eliminated to achieve a safe domestic environment” (30). This perception of microbes as bad actors can negatively impact human health, just as it can negatively impact soil health. For instance, research demonstrated that use of germicidal soaps reduces populations of protective microbes living on human skin, increasing risk of skin cancer (78).
This societal microbiophobia extends to farmers and impacts their land management decisions. Farmers are trained in standardized industrial knowledge that myopically focuses on eradicating bad actor microbes using agrochemical cocktails while largely overlooking beneficial microbes. Often, a farmer’s decision to habitually apply pesticides is “not based on economic rationale, but instead guided by worst case scenarios, molded by loss aversion, shaped by peer pressure or triggered through marketing campaigns by agrochemical suppliers” (79). For instance, Rod Koda, a strawberry grower in Watsonville, California, pre-emptively used methyl bromide each season to ward off potential soil pathogens (80). He is haunted by stories from the past—in the late 1950s red stele, a fungal pathogen that can linger in the soil for a decade or more, swept through the region, forcing farmers to abandon their land. Farmers are afraid of what may “lurk in the dirt” (80)—and recent agricultural history gives them plenty of fodder to feed this fear.
In a similar fashion, farmers are fearful of insects that devastate crops and use insecticides to manage insect pest populations. Similar to fumigants and other pesticides targeting soil microbes, insecticides kill target and non-target organisms, some of which are beneficial, such as pollinators or natural enemies of pests. In a comprehensive review of farmer knowledge of insects, Wyckhuys et al. 2019 reported that up to 70 percent of growers surveyed were unaware of natural enemies or unfamiliar with the concept of biological control. Farmer ecological literacy was particularly shallow for smaller pests, such as mites (79). This is an extension of the trend we see with microbes—it is hard to understand the importance invisible worlds on visible worlds. Although agricultural pests consist of only a small fraction of total insect biodiversity (for instance in rice ecosystems, seventeen percent of the total arthropod community are herbivores, and one percent of all herbivores are considered pests as compared to 64 percent of herbivores which are considered natural enemies), farmers focus their energy and attention on controlling pest communities, all but ignoring the remaining insects. Again, this myopic focus on bad actor organisms is implanted by standardized industrial agriculture knowledge creating systems which seek to simplify and control biology.
The limited research that exists suggests farmer ecological literacy concerning belowground microbial diversity is even more deficient than farmer insect literacy. Pauli et al. 2016 performed the first synthesis of high-quality research reporting farmer knowledge of soil organisms, reviewing a total of 60 studies. While there has been much written about farmer knowledge of chemical and physical soil properties, there is a dearth of research reporting farmer soil biological knowledge. There is even less reported on farmer knowledge of non-visible soil fauna—the most commonly mentioned taxonomic groups across surveyed studies were earthworms, followed by termites, beetles, and ants (51).
It is important to note that these reviews–Wyckhuys et al. 2019 and Pauli et al. 2016–characterizing farmer ecological literacy of insects and soil fauna respectively are limited in their scope in two important ways: (1) The vast majority of the studies reviewed focus on developing countries and smallholder agricultural systems. There is limited documentation of large-scale farmer knowledge of insects or soil fauna in developed countries such as the United States. (2) Neither of these studies sufficiently cover farmer knowledge of organisms that are invisible to the naked eye. Of the 60 studies surveyed by Pauli et al. 2016, only six mentioned non-visible soil microbes. Pauli et al. 2016 intended at the onset of the synthesis to characterize farmer knowledge of all microbes (visible and invisible), but, in the end, the available data limited the review to visible fauna.
These gaps and biases in farmer knowledge of microbes in soil systems are due at least in part to technological constraints. We have only recently established the high-resolution molecular technology needed study the complexity of belowground soil microbiomes. Unlike soil biology, we have had the technology to assess the physical and chemical properties of soil for many decades. But technological constraint is not the sole reason that standardized farmer education concerning soil continues to focus on the physical and chemical properties of soil while ignoring soil biology. This focus on physical and chemical characteristics is part of the larger simplifying project of agrilogistics. As Dr. Kris Nichols, a mycologist and former Chief Scientist at the Rodale Institute, adeptly observed, “agriculture, especially in the last 20 to 30 years, has become someone handing you a cookbook and telling you, as a farmer, exactly what to do and you don’t have to think about it” (81). This one size fits all approach of agrilogistics discourages complexity, biodiversity, and farmer ecological literacy. It is the antithesis of place-based agriculture advocated by natural systems thinkers like Wendel Berry and Wes Jackson (82, 83).
Taken together, this shallow ecological literacy emerging from agrologists (characterized by Wyckhuys et al. 2019 and Pauli et al. 2016) intensifies farmer reliance on standardized solutions offered by agroindustrial models of production, making them beholden to corporate agendas. Agrochemical suppliers seek to benefit from farmer ecological illiteracy, since decreased literacy traps farmers in pesticide treadmills. Overall, this trend of farmer ‘deskilling’ (84) diminishes farmer performance, innovation, and satisfaction, while simultaneously degrading the biodiversity and resilience of their land (84). It is a cycle that must end.
Video Summary of Project
References
- W. B. Logan, Dirt: the ecstatic skin of the earth (W.W. Norton & Company, 2007).
- T. E. Crews, W. Carton, L. Olsson, Is the future of agriculture perennial? Imperatives and opportunities to reinvent agriculture by shifting from annual monocultures to perennial polycultures. Global Sustainability 1 (2018).
- M. E. Schipanski, et al., Realizing Resilient Food Systems. BioScience 66, 600–610 (2016).
- T. Morton, Dark ecology: for a logic of future coexistence (Columbia University Press, 2016) (January 22, 2020).
- J. A. Foley, et al., Solutions for a cultivated planet. Nature 478, 337–342 (2011).
- P. Grassini, K. M. Eskridge, K. G. Cassman, Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat Commun 4 (2013).
- Richard. Heinberg, Peak everything: waking up to the century of declines (New Society Publishers, 2007).
- A. G. Power, Ecosystem services and agriculture: tradeoffs and synergies. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 2959–2971 (2010).
- Ç. H. Şekercioğlu, et al., Long-term declines in bird populations in tropical agricultural countryside. PNAS 116, 9903–9912 (2019).
- P. M. Kopittke, N. W. Menzies, P. Wang, B. A. McKenna, E. Lombi, Soil and the intensification of agriculture for global food security. Environment International 132, 105078 (2019).
- G. Govers, R. Merckx, B. van Wesemael, K. Van Oost, Soil conservation in the 21st century: why we need smart agricultural intensification. SOIL 3, 45–59 (2017).
- J. Sanderman, T. Hengl, G. J. Fiske, Soil carbon debt of 12,000 years of human land use. PNAS 114, 9575–9580 (2017).
- D. 1925- Pimentel, Marcia. Pimentel, Food, energy, and society, Revised edition. (University Press of Colorado, 1996).
- J. N. Galloway, et al., Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 70, 153–226 (2004).
- N. N. Rabalais, et al., Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 7, 585–619 (2010).
- P. S. Jørgensen, et al., Antibiotic and pesticide susceptibility and the Anthropocene operating space. Nature Sustainability 1, 632–641 (2018).
- J. Clapp, J. Purugganan, Contextualizing corporate control in the agrifood and extractive sectors. Globalizations 17, 1265–1275 (2020).
- E. S. Bernhardt, E. J. Rosi, M. O. Gessner, Synthetic chemicals as agents of global change. Frontiers in Ecology and the Environment 15, 84–90 (2017).
- T. A. Arcury, et al., Pesticide exposure among Latinx children: Comparison of children in rural, farmworker and urban, non-farmworker communities. Science of The Total Environment 763, 144233 (2021).
- J. R. Rohr, et al., Emerging human infectious diseases and the links to global food production. Nat Sustain 2, 445–456 (2019).
- M. G. A. V. D. Heijden, R. D. Bardgett, N. M. V. Straalen, The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters 11, 296–310 (2008).
- R. Cavicchioli, et al., Scientists’ warning to humanity: microorganisms and climate change. Nature Reviews Microbiology 17, 569–586 (2019).
- M. A. Tsiafouli, et al., Intensive agriculture reduces soil biodiversity across Europe. Global Change Biology 21, 973–985 (2015).
- C. W. Fernandez, P. G. Kennedy, Moving beyond the black-box: fungal traits, community structure, and carbon sequestration in forest soils. New Phytol. 205, 1378–1380 (2015).
- S. F. Bender, C. Wagg, M. G. A. van der Heijden, An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends in Ecology & Evolution 31, 440–452 (2016).
- D. H. Wall, U. N. Nielsen, J. Six, Soil biodiversity and human health. Nature 528, 69–76 (2015).
- N. Fierer, Embracing the unknown: disentangling the complexities of the soil microbiome. Nature Reviews Microbiology 15, 579–590 (2017).
- U. De Corato, Soil Microbiome Manipulation Gives New Insights in Plant Disease-Suppressive Soils from the Perspective of a Circular Economy: A Critical Review. Sustainability 13, 10 (2021).
- R. D. Bardgett, W. H. van der Putten, Belowground biodiversity and ecosystem functioning. Nature 515, 505–511 (2014).
- K. Timmis, et al., The urgent need for microbiology literacy in society. Environmental Microbiology 21, 1513–1528 (2019).
- C. A. Guerra, et al., Blind spots in global soil biodiversity and ecosystem function research. Nature Communications 11, 3870 (2020).
- J. R. Marchesi, J. Ravel, The vocabulary of microbiome research: a proposal. Microbiome 3, 31 (2015).
- G. Berg, et al., Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 103 (2020).
- J. Whipps, K. Lewis, R. Cooke, Mycoparasitism and plant disease control. Manchester University Press, 161–187 (1988).
- A. Dubey, et al., Soil microbiome: a key player for conservation of soil health under changing climate. Biodivers Conserv 28, 2405–2429 (2019).
- P. A. H. M. Bakker, et al., The Soil-Borne Identity and Microbiome-Assisted Agriculture: Looking Back to the Future. Mol. Plant. 13, 1394–1401 (2020).
- E. Rosenberg, I. Zilber-Rosenberg, Microbes Drive Evolution of Animals and Plants: the Hologenome Concept. mBio 7 (2016).
- I. Zachar, G. Boza, Endosymbiosis before eukaryotes: mitochondrial establishment in protoeukaryotes. Cell. Mol. Life Sci. 77, 3503–3523 (2020).
- W. Remy, T. N. Taylor, H. Hass, H. Kerp, Four hundred-million-year-old vesicular arbuscular mycorrhizae. PNAS 91, 11841–11843 (1994).
- K. A. Pirozynski, D. W. Malloch, The origin of land plants: a matter of mycotrophism. BioSystems 6, 153–164 (1975).
- A. Pascale, S. Proietti, I. S. Pantelides, I. A. Stringlis, Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion. Front. Plant Sci. 10 (2020).
- H. P. Bais, T. L. Weir, L. G. Perry, S. Gilroy, J. M. Vivanco, The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266 (2006).
- T. J. Thirkell, M. D. Charters, A. J. Elliott, S. M. Sait, K. J. Field, Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. Journal of Ecology 105, 921–929 (2017).
- R. L. Berendsen, C. M. J. Pieterse, P. A. H. M. Bakker, The rhizosphere microbiome and plant health. Trends in Plant Science 17, 478–486 (2012).
- C. Sánchez-Cañizares, B. Jorrín, P. S. Poole, A. Tkacz, Understanding the holobiont: the interdependence of plants and their microbiome. Current Opinion in Microbiology 38, 188–196 (2017).
- S. R. Bordenstein, K. R. Theis, Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes. PLOS Biology 13, e1002226 (2015).
- C. A. Guerra, et al., Tracking, targeting, and conserving soil biodiversity. Science 371, 239–241 (2021).
- T. E. Sackett, A. T. Classen, N. J. Sanders, Linking soil food web structure to above- and belowground ecosystem processes: a meta-analysis. Oikos 119, 1984–1992 (2010).
- P. Visconti, et al., Protected area targets post-2020. Science 364, 239–241 (2019).
- B. K. Trevelline, S. S. Fontaine, B. K. Hartup, K. D. Kohl, Conservation biology needs a microbial renaissance: a call for the consideration of host-associated microbiota in wildlife management practices. Proc Biol Sci 286 (2019).
- Pauli N., Abbott L.K., Negrete-Yankelevich S., Andres P., Farmers’ knowledge and use of soil fauna in agriculture: A worldwide review. Ecology and Society 21 (2016).
- D. H. Wall, R. D. Bardgett, E. Kelly, Biodiversity in the dark. Nature Geoscience 3, 297–298 (2010).
- M. Blackwell, The fungi: 1, 2, 3 … 5.1 million species? Am J Bot 98, 426–438 (2011).
- D. L. Hawksworth, R. Lücking, Fungal Diversity Revisited: 2.2 to 3.8 Million Species. Microbiol Spectr 5 (2017).
- E. N. Lughadha, et al., Extinction risk and threats to plants and fungi. PLANTS, PEOPLE, PLANET 2, 389–408 (2020).
- M. Gopal, A. Gupta, Building plant microbiome vault: a future biotechnological resource. Symbiosis 77, 1–8 (2019).
- E. Verbruggen, T. Kiers, Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol Appl 3, 547–560 (2010).
- S. Banerjee, et al., Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. The ISME Journal 13, 1722–1736 (2019).
- Z. Dai, et al., Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob Chang Biol 24, 3452–3461 (2018).
- L. Feld, et al., Pesticide Side Effects in an Agricultural Soil Ecosystem as Measured by amoA Expression Quantification and Bacterial Diversity Changes. PLOS ONE 10, e0126080 (2015).
- M. Lupatini, G. W. Korthals, M. de Hollander, T. K. S. Janssens, E. E. Kuramae, Soil Microbiome Is More Heterogeneous in Organic Than in Conventional Farming System. Front Microbiol 7 (2017).
- F. T. de Vries, et al., Soil bacterial networks are less stable under drought than fungal networks. Nature Communications 9, 3033 (2018).
- M. A. Hassaan, A. El Nemr, Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. The Egyptian Journal of Aquatic Research 46, 207–220 (2020).
- F. Sanchez-Bayo, K. A. G. Wyckhuys, Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 232, 8–27 (2019).
- M. L. Eng, B. J. M. Stutchbury, C. A. Morrissey, A neonicotinoid insecticide reduces fueling and delays migration in songbirds. Science 365, 1177–1180 (2019).
- P. Mineau, M. Whiteside, Pesticide acute toxicity is a better correlate of U.S. grassland bird declines than agricultural intensification. PLoS ONE 8, e57457 (2013).
- M. A. Beketov, B. J. Kefford, R. B. Schäfer, M. Liess, Pesticides reduce regional biodiversity of stream invertebrates. PNAS 110, 11039–11043 (2013).
- C. A. Brühl, T. Schmidt, S. Pieper, A. Alscher, Terrestrial pesticide exposure of amphibians: An underestimated cause of global decline? Scientific Reports 3, 1135 (2013).
- E. H. Berheim, et al., Effects of Neonicotinoid Insecticides on Physiology and Reproductive Characteristics of Captive Female and Fawn White-tailed Deer. Sci Rep 9, 1–10 (2019).
- C. Nerozzi, et al., Effects of Roundup and its main component, glyphosate, upon mammalian sperm function and survival. Scientific Reports 10, 11026 (2020).
- C. Gasnier, et al., Glyphosate-based herbicides are toxic and endocrine disruptors in human cell lines. Toxicology 262, 184–191 (2009).
- Garry Vincent F, et al., Birth defects, season of conception, and sex of children born to pesticide applicators living in the Red River Valley of Minnesota, USA. Environmental Health Perspectives 110, 441–449 (2002).
- R. P. Coullery, M. E. Ferrari, S. B. Rosso, Neuronal development and axon growth are altered by glyphosate through a WNT non-canonical signaling pathway. NeuroToxicology 52, 150–161 (2016).
- N. Wan, G. Lin, Parkinson’s Disease and Pesticides Exposure: New Findings From a Comprehensive Study in Nebraska, USA. The Journal of Rural Health 32, 303–313 (2016).
- C. A. Brühl, J. G. Zaller, Biodiversity Decline as a Consequence of an Inappropriate Environmental Risk Assessment of Pesticides. Front. Environ. Sci. 7 (2019).
- Soil Health Institute, Living Soil Film (2018) (April 2, 2021).
- A. Hartmann, M. Rothballer, M. Schmid, Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 312, 7–14 (2008).
- T. Nakatsuji, et al., A commensal strain of Staphylococcus epidermidis protects against skin neoplasia. Science Advances 4, eaao4502 (2018).
- K. a. G. Wyckhuys, et al., Ecological illiteracy can deepen farmers’ pesticide dependency. Environ. Res. Lett. 14, 093004 (2019).
- B. Yeung, T. Kendall, D. Andrew, California’s strawberry industry is hooked on dangerous pesticides. Reveal from The Center of Investigative Reporting (2014) (February 24, 2021).
- , Microorganisms Build Fertility and Mitigate Climate Change: An Interview with Dr. Kris Nichols. Bioneers (2020) (April 2, 2021).
- W. 1934- Berry, The unsettling of America: culture & agriculture (Counterpoint, 2015) (April 2, 2021).
- Wes. Jackson, New roots for agriculture, New ed. (University of Nebraska Press, 1985) (April 2, 2021).
- G. D. Stone, Agricultural Deskilling and the Spread of Genetically Modified Cotton in Warangal. Current Anthropology 48, 67–103 (2007).
Image Citations
Image 1. Intensive Industrial Agriculture
Photograph. Public Domain.
Image 2. A Handful of Healthy Soil
Alex Lintner. Photograph. May 2021. Attribution CC BY-NC 2.0.
Image 3. Mycorrhizal Fungi in a Plant Root
Alex Lintner. Microscope Image. April 2019. Attribution CC BY-NC 2.0.
Image 4. Industrial Agriculture Simplifies Soil Microbiomes
Alex Lintner. Illustration. BioRender.com. May 2021. Attribution CC BY-NC 2.0.
Image 5. Fumigants in Use
Benketaro. Photograph. October 2009. Attribution CC BY 2.0.

