Working with AMF on the Farm
Key Takeaways
In this module, you will learn:
- which on-farm practices benefit AMF
- how to cultivate your own AMF inoculum
- two practices for monitoring AMF on your farm
As we have seen in previous modules, arbuscular mycorrhizal fungi (AMF) are powerful plant allies with broad applications in agriculture.
AMF colonize the majority of crop plants.
On-farm Practices the Benefit AMF
There are many on-farm practices that support AMF soil populations.
Five practices that promote AMF populations in the living soil:
Practice 1: Maintain living roots in the soil year-round
Remember, AMF need to colonize a living plant root in order to survive. By having roots in the ground, you are providing AMF with a consistent food source. You can maintain living roots in the soil by incorporating cover crops or perennial crops.
Practice 2: Reduce tillage
Tillage physically rips up AMF mycelium in the living soil. By reducing tillage or by going no-till you are supporting AMF populations.
Practice 3: Reduce or eliminate fertilizer input
Remember, AMF do not confer a competitive advantage to the plant if the plant has easy access to phosphorous or nitrogen from fertilizers. Fertilizers outsource the job of AMF.
Practice 4: Increase plant biodiversity
More diverse plants on your farm translate to more diverse soil fungal populations. Different plants offer fungi different types of sugars.
Practice 5: Reduce or eliminate the use of pesticides
Pesticides disrupt the balance of fungal populations. Fungicides can wipe out all fungi in the living soil.
How to innoculate AMF on the Farm
A more direct way of supporting AMF in the soil is to inoculate the soil with AMF.
AMF inoculation can help minimize farm inputs and reduce the cost of producing health crops. AMF inoculation can further support farm profits by directly increasing yields.
There are many AMF inocula commercially available, but farmers can grow a potent inoculum of the AMF indigenous to their farms using an inexpensive and reliable method.
The On-Farm AMF Inoculum Production System was developed by a partnership between the The Rodale Institute and the USDA-Agricultural Research Service.
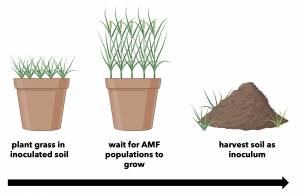
The Production System
The system uses native soil to inoculate the host plant with indigenous AMF. As the host plant grows, AMF populations from the native soil proliferate. The following spring, you harvest the spore and propagule filled soil and use it as potent inoculum.
This protocol suggests that you use bahiagrass. It is a dependable host for the majority of AMF species.
Image 1. Production System
How to make your own AMF inoculum
Step 1: Germination
Germinate the bahiagrass seeds.
Step 2: Inoculation
After the last frost, transplant the grass into grow bags with “inoculum starter.” The starter is just soil from your farm. To obtain a diverse array of AMF, it is suggested that you take soil samples from natural areas of your farm.
Step 3: Growth
Grow the grass in the pot with the inoculum starter. Water and weed as needed. The AMF from the starter will proliferate throughout the growing season.
Step 4: Death
Let the host plant die from frost. The AMF will overwinter.
Step 5: Harvest
The following spring, harvest your soil in the grow bags. The soil and plant roots can now serve as inoculum. The soil and roots will be full of AMF spores, hyphae, and vesicles.
Monitoring AMF Populations on the Farm
You can track changes in AMF communities as you change farm management practices.
On-farm monitoring of soil biology builds local knowledge.
Below, you will find two simple and cost-effective methods to assess the soil fauna on your farm.
Monitoring AMF Spores
You can get a good idea of the quantity and diversity of AMF in your soil by looking at the spores. AMF spores come in a variety of colors and sizes. AMF spores are much larger than other fungal spores, making them easy to isolate and quantify.
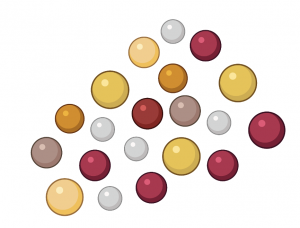
Image 2. Diverse AMF Spores
The International Culture Collection of Vesicular Arbuscular Mycorrhizal Fungi (INVAM) at West Virginia University has a collection of helpful online resources.
They outline a protocol for sieving soil and harvesting AMF spores. It also guides you through how to quantify spores.

Image 3. Sieve Soil for Spores
Staining Roots for Mycorrhizal Fungi
Another way to quantify AMF colonization is staining your crop roots for mycorrhizal fungi. After staining you can visualize and quantify AMF colonization using a light microscope.
The On-Farm Soil Monitoring Project is a join project led by Wheatbelt Natural Resource Management, the South West Catchments Council, and The University of Western Australia. They have developed an open access On Farm Soil Monitoring Handbook. The staining instructions below are based on the instructions outlined in the handbook in Chapter 3 and Chapter 5.
How to stain roots for AMF
Step 1: assemble the required materials
- Forceps
- Small flat plastic or glass dishes
- Small glass or plastics bottles with caps
- Potassium hydroxide (KOH) (safety advice – avoid skin contact with KOH)
- Vinegar
- Black Ink
- Plant roots
- Light microscope (compound)

Step 2: prepare the following solutions
- 10% KOH (10 g KOH in 100 ml water) (safety advice – avoid skin contact with KOH)
- 5% vinegar (5 mL vinegar + 95 mL water)
- 5% black ink (5 mL black ink + 95 mL of 5% vinegar)
Step 3: prepare the roots for staining by clearing with KOH
- Collect and clean plant roots w/ tap water and cut into ~2 cm pieces.
- Place roots in bottle and cover with 10% KOH. Leave roots in 10% KOH for 5 to 7 days at room temperature.
- After roots are cleared by 10% KOH, rinse roots with water several times.
Step 4: stain the AMF in the roots
- Rinse roots with 5% vinegar one time.
- Add roots to 5% black ink and leave overnight.
- After the roots are stained by 5% black ink, rinse roots with water once.
Step 5: visualize the AMF in the roots
- Place the stained roots in flat dish and cover with water.
- View roots with light microscope.
- Root sample can be stored in water for several weeks
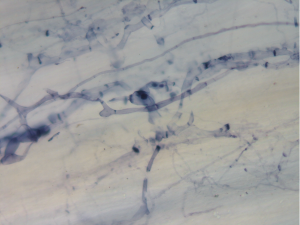
Image 4. Mycorrhizal Fungi in Plant Roots
Informational Handout
Simple On-Farm Protocol for Staining Plant Roots for Mycorrhizal Fungi
Link to PFD: staining protocol handout

Image Citations
Image 1. Production System
Alex Lintner. Illustration. BioRender.com. May 2021. Attribution CC BY-NC 2.0.
Image 2. Diverse AMF Spores
Alex Lintner. Illustration. BioRender.com. May 2021. Attribution CC BY-NC 2.0.
Image 3. Sieve Soil for Spores
Alex Lintner. Photograph. April 2019. Attribution CC BY-NC 2.0.
Image 4. Mycorrhizal Fungi in Plant Roots
Alex Lintner. Microscope Photograph. April 2019. Attribution CC BY-NC 2.0.