4.3 Dysbiosis and Depression
Hannan Algitami
Introduction
As discussed in earlier chapters, the bidirectional relationship between the gut and the brain, or the gut-brain-axis, raises many questions about how brain function is affected by the gut and vice versa. More specifically, the degree to which the gut microbiome is implicated in mental illness has only recently been explored.
Mood disorders such as MDD (Major Depressive Disorder) are idiopathic and have not had a successful targeted treatment mainly because they affect many areas. One of the speculated targets of these disorders is the gut due to the high rates of comorbidity between mental illness and gastrointestinal illness. For instance, many people suffering from IBS (Irritable Bowel Syndrome) also suffer from MDD, with antidepressants being one of the only medications successful in treating IBS. Therefore, there is a clear connection between the gut and mental illness; however, which system is affected first and hence which imbalance leads to the other is yet to be elucidated (Rogers et al., 2016).
To solve this conundrum, researchers from far and wide have attempted to find a relationship between gastrointestinal imbalance and MDD. What was known thus far was that MDD is comorbid with many gastrointestinal disorders and that antidepressants somehow affect the gut and serve as treatments for these disorders. They hence proposed that the gut microbiota must be affected by MDD, which is why antidepressants, acting in most cases to reverse the condition, also bring the MDD-induced changes in the gut back to homeostasis. All of these were mere conjectures and therefore had to be followed up by more investigation.
Dysbiosis and depression
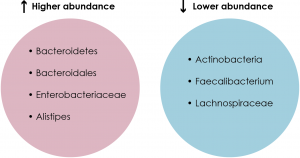
The first area that needed to be addressed was the gut microbial profiles of MDD patients and healthy controls. Comparative analyses using techniques such as 16S rRNA sequencing revealed a significant difference between the gut microbial populations of the two groups (See Figure 1). For example, one study found that MDD patients had a higher relative abundance of Bacteroidetes and a lower relative abundance of Actinobacteria compared to healthy controls (see Chapter 4.1 for details on bacterial phyla) (Zheng et al., 2016a). In addition, another study found that patients with MDD had higher levels of Enterobacteriaceae and Alistipes but lower levels of Faecalibacterium compared to healthy controls (Jiang et al., 2015; Liu, 2017). Moreover, a third study noted higher levels of Bacteroidales but lower levels of Lachnospiraceae in MDD patients compared to healthy controls (Liu, 2017; Naseribafrouei et al., 2014). Hence, there is indeed a relationship between the MDD pathology and the gut microbiome, but the direction in which this relationship goes is unknown.
To attempt to find out the direction of this complex relationship, researchers manipulated the gut microbiome composition in a number of ways and observed the behavioural and physiological changes that occurred, if any. Wiping out the microbiome completely using antibiotics has been used as a way to gauge the importance of gut bacteria on behaviour; however, this has come under much scrutiny especially after many studies have shown antibiotics to have vastly acting effects, introducing many confounds. Specifically, antibiotics are able to alter the microbiome by either depleting it, increasing the number of antibiotic-resistant bacteria or affecting the host tissue directly. Antibiotic-induced effects on host tissue may extend to the CNS (Central Nervous System), potentially having a neuroactive effect. All of these may separately affect mental health and therefore make it difficult to attribute results merely to microbiota (Champagne-Jorgensen et al., 2019; Flux & Lowry, 2020; Morgun et al., 2015).
Psychobiotics
Enriching the gut microbiome using agents like prebiotics and probiotics has also been widely explored. Probiotics are live microorganisms found in fermented foods that, when ingested, are believed to enrich the gut microbiome. They have been proposed as a treatment for depression due to their supposed ability to improve gut health. Even though they have shown promise as therapeutic agents or as accompaniments to traditional antidepressants by alleviating symptoms of those with depression, studies have shown mixed results and a definitive claim about their efficacy cannot yet be made (Flux & Lowry, 2020; Nadeem et al., 2019). Although studies on probiotics have often focused on individuals with pre-existing pathologies, supplements given to healthy adults were shown to have only a transient effect on the gut microbiome. Hence, their lack of universal effect calls to question their mode of action and necessitates that more studies be done on more diverse samples in order to elucidate whether they can be used as a treatment for different pathologies affecting the gut, such as depression (Khalesi et al., 2018).
Furthermore, Prebiotics are substances that can be broken down by the gut microbiota for which humans do not have the enzymes to metabolize (Flux & Lowry, 2020; Holscher, 2017). An experiment done on chronic-stress mice showed that prebiotic administration affected SCFA (Short Chain Fatty Acid) levels in ways that are correlated with a positive behavioural state. It also reduced stress hormone levels, reduced pro-inflammatory cytokine levels and had anti-depressant and anxiolytic effects (Burokas et al., 2017; Flux & Lowry, 2020; Kao et al., 2016; Louis et al., 2016). These results show that prebiotic treatment may have a positive effect on depression; however, as with probiotics, more studies need to be performed in order to make a strong claim for its effectiveness.
Prebiotics and Probiotics can be combined together under the more modern term “psychobiotics” which has been recently coined to refer to any bacterial agent that affects mental health. However, there are often mixed results about how these nutrients affect mental health and behaviour. Therefore, further studies using larger samples and more human trials need to be done to clarify this idea (Flux & Lowry, 2020; Sarkar et al., 2016).
Microbiota Transfer
Another technique that has been used to study the relationship between dysbiosis and depression is FMT (Fecal Microbiota Transfer). In this technique, fecal samples are extracted from a donor and administered to a recipient in various forms. In gut microbiome research, FMT is done by transferring human fecal samples to GF (Germ-Free) or SPF (Specific Pathogen Free) mice in order to create disease models. A number of studies have used this technique to examine the gut-related effects of various disorders, including MDD. In one study, fecal samples were collected from either MDD patients or healthy controls and administered to healthy GF mice. The results showed that mice receiving MDD-derived microbiota showed depressive-like behaviours, such as higher immobility time in locomotor tests as well as disturbances in metabolic activity among other behavioural and physiological symptoms of the MDD pathology (Zheng et al., 2016b). This shows that microbiota transfer alone can induce depression in otherwise healthy mice.
Since FMT has been shown to induce depression, the question of whether it can be used to reverse the condition arises. If this technique is effective in altering gut microbiota composition, depression-induced dysbiosis should be attenuated with a healthy microbiota transfer; however, this has not yet been tested. Moreover, although FMT has been viewed as a potential treatment for many pathologies such as ASD (Autism Spectrum Disorder), it is still in its infancy and has not yet been widely tested in humans (Kang et al., 2017). In addition to this, the natural variation in the human gut microbiome makes it difficult to standardize a singular sample of healthy microbiota to be used for transplantation (Flux & Lowry, 2020; Human Microbiome Project Consortium, 2012). Hence, whether FMT can be used as a potential treatment for MDD and other such conditions is still in question and whether it will be viable and generalizable to humans is another point to consider.
A Multifaceted disorder
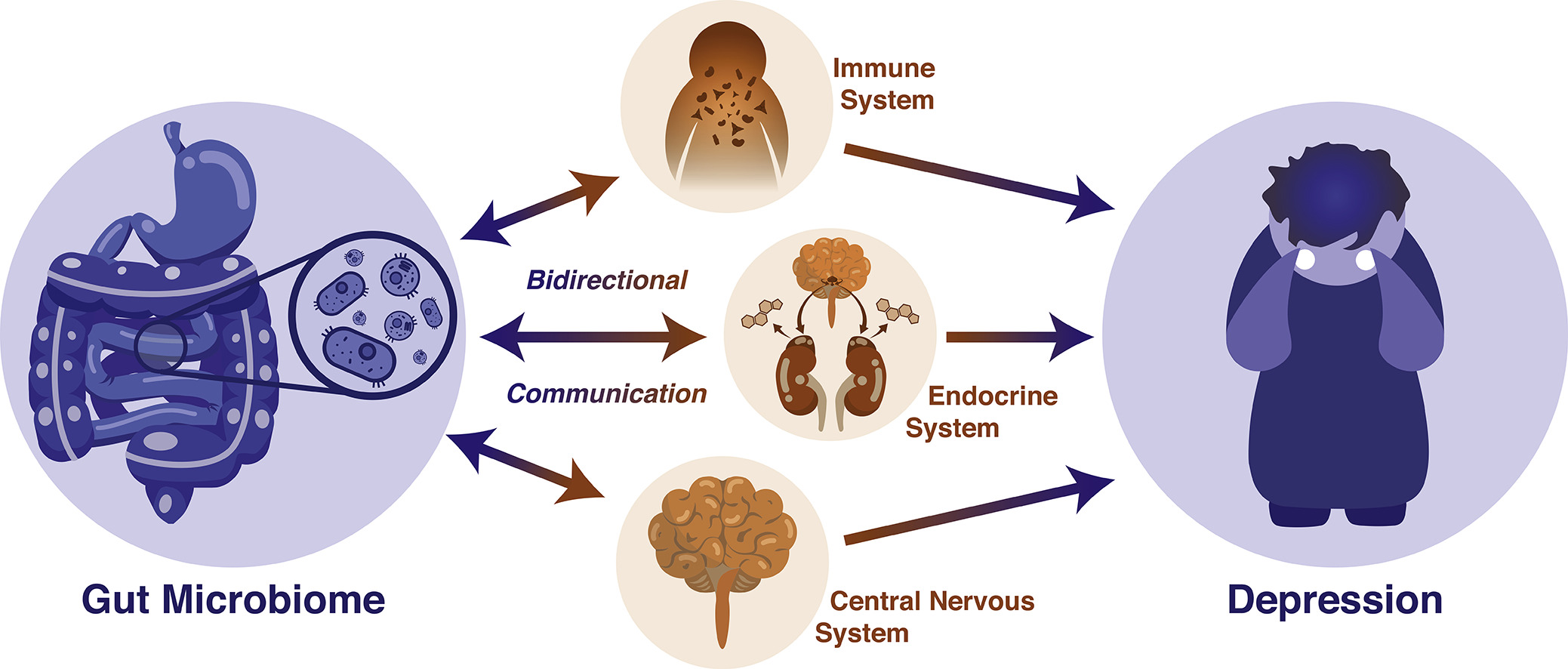
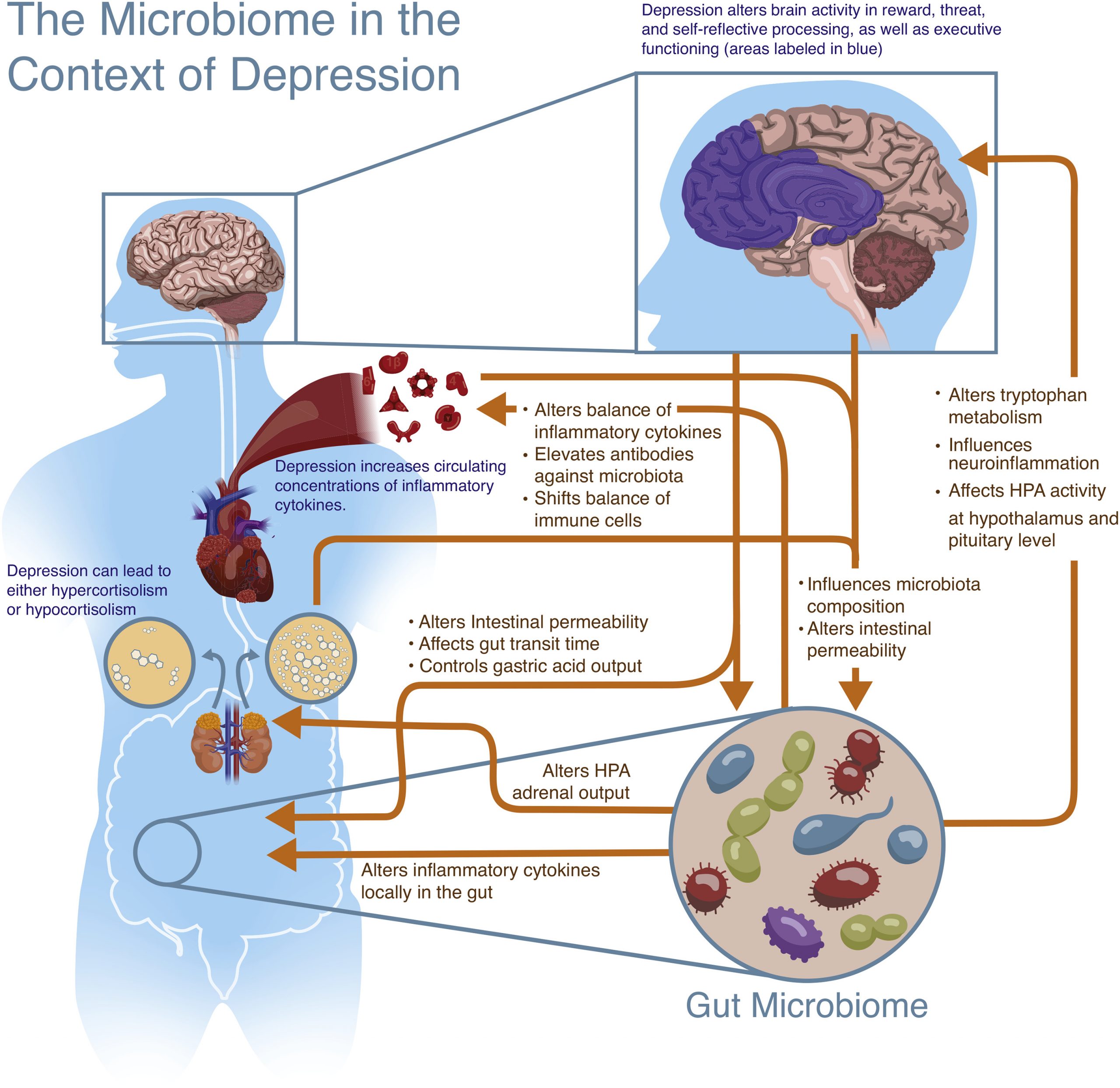
Like the gut-brain axis, a similar highway known as the microbiome-gut-brain-axis is thought to be a bidirectional form of communication between the microbiota and the CNS, ultimately influencing behaviour. Although the research that has been outlined above shows a very strong connection between depression and dysbiosis, a definitive order in which the imbalances occur is difficult to be understood in isolation. Rather, the interplay between the gut microbiome and depression should be thought of as a two-way communication that is dependent on many other systems and factors along the way. Hence, the gut microbiome may influence the CNS directly by way of its metabolites such as SCFAs and cytokines travelling through the vagus nerve, or indirectly by way of the enteric system. Conversely, the CNS may release factors that influence the gut, intestinal permeability and microbiota composition (See Figures 2&3). This entire system and the communication between these two components, the gut microbiome and the brain, allow for a nuanced manifestation of depression that cannot be meaningfully understood unless a comprehensive outlook is taken (Flux & Lowry, 2020).
Challenging Questions
1. Design an experiment to investigate the directionality of the microbiome-gut-brain axis and its implication in depression.
2. How can the gut microbiome be used as a potential treatment for depression and what are some caveats to the gut-related treatments proposed above?
3. What is an effective way to address the imbalances in the gut induced by depression in the already existing treatment techniques?
Keywords
Gut-brain-axis; MDD; Probiotics; Prebiotics; Psychobiotics; FMT; Microbiome-gut-brain-axis
References
Burokas, A., Arboleya, S., Moloney, R. D., Peterson, V. L., Murphy, K., Clarke, G., Stanton, C., Dinan, T. G., & Cryan, J. F. (2017). Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biological Psychiatry, 82(7), 472–487. https://doi.org/10.1016/j.biopsych.2016.12.031
Champagne-Jorgensen, K., Kunze, W. A., Forsythe, P., Bienenstock, J., & McVey Neufeld, K.-A. (2019). Antibiotics and the nervous system: More than just the microbes? Brain, Behavior, and Immunity, 77, 7–15. https://doi.org/10.1016/j.bbi.2018.12.014
Flux, M. C., & Lowry, C. A. (2020). Finding intestinal fortitude: Integrating the microbiome into a holistic view of depression mechanisms, treatment, and resilience. Neurobiology of Disease, 135, 104578. https://doi.org/10.1016/j.nbd.2019.104578
Holscher, H. D. (2017). Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes, 8(2), 172–184. https://doi.org/10.1080/19490976.2017.1290756
Human Microbiome Project Consortium. (2012). Structure, function and diversity of the healthy human microbiome. Nature, 486(7402), 207–214. https://doi.org/10.1038/nature11234
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., Wang, W., Tang, W., Tan, Z., Shi, J., Li, L., & Ruan, B. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity, 48, 186–194. https://doi.org/10.1016/j.bbi.2015.03.016
Kang, D.-W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., Khoruts, A., Geis, E., Maldonado, J., McDonough-Means, S., Pollard, E. L., Roux, S., Sadowsky, M. J., Lipson, K. S., Sullivan, M. B., Caporaso, J. G., & Krajmalnik-Brown, R. (2017). Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome, 5(1), 10. https://doi.org/10.1186/s40168-016-0225-7
Kao, A. C. C., Harty, S., & Burnet, P. W. J. (2016). The Influence of Prebiotics on Neurobiology and Behavior. International Review of Neurobiology, 131, 21–48. https://doi.org/10.1016/bs.irn.2016.08.007
Khalesi, S., Bellissimo, N., Vandelanotte, C., Williams, S. L., Stanley, D., & Irwin, C. (2018). A review of probiotic supplementation in healthy adults: Helpful or hype? European Journal of Clinical Nutrition. https://doi.org/10.1038/s41430-018-0135-9
Liu, R. T. (2017). The microbiome as a novel paradigm in studying stress and mental health. The American Psychologist, 72(7), 655–667. https://doi.org/10.1037/amp0000058
Louis, P., Flint, H. J., & Michel, C. (2016). How to Manipulate the Microbiota: Prebiotics. Advances in Experimental Medicine and Biology, 902, 119–142. https://doi.org/10.1007/978-3-319-31248-4_9
Morgun, A., Dzutsev, A., Dong, X., Greer, R. L., Sexton, D. J., Ravel, J., Schuster, M., Hsiao, W., Matzinger, P., & Shulzhenko, N. (2015). Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut, 64(11), 1732–1743. https://doi.org/10.1136/gutjnl-2014-308820
Nadeem, I., Rahman, M. Z., Ad-Dab’bagh, Y., & Akhtar, M. (2019). Effect of probiotic interventions on depressive symptoms: A narrative review evaluating systematic reviews. Psychiatry and Clinical Neurosciences, 73(4), 154–162. https://doi.org/10.1111/pcn.12804
Naseribafrouei, A., Hestad, K., Avershina, E., Sekelja, M., Linløkken, A., Wilson, R., & Rudi, K. (2014). Correlation between the human fecal microbiota and depression. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society, 26(8), 1155–1162. https://doi.org/10.1111/nmo.12378
Rogers, G. B., Keating, D. J., Young, R. L., Wong, M.-L., Licinio, J., & Wesselingh, S. (2016). From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Molecular Psychiatry, 21(6), 738–748. https://doi.org/10.1038/mp.2016.50
Sarkar, A., Lehto, S. M., Harty, S., Dinan, T. G., Cryan, J. F., & Burnet, P. W. J. (2016). Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends in Neurosciences, 39(11), 763–781. https://doi.org/10.1016/j.tins.2016.09.002
Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., Zeng, L., Chen, J., Fan, S., Du, X., Zhang, X., Yang, D., Yang, Y., Meng, H., Li, W., Melgiri, N. D., Licinio, J., Wei, H., & Xie, P. (2016a). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Molecular Psychiatry, 21(6), 786–796. https://doi.org/10.1038/mp.2016.44
Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., Zeng, L., Chen, J., Fan, S., Du, X., Zhang, X., Yang, D., Yang, Y., Meng, H., Li, W., Melgiri, N. D., Licinio, J., Wei, H., & Xie, P. (2016b). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Molecular Psychiatry, 21(6), 786–796. https://doi.org/10.1038/mp.2016.44

